Introduction
This page contains information on education, training, CPD, and miscellaneous support materials for pharmacy professionals, including our 'Genomics in Practice' newsletter for pharmacists and pharmacy professionals which are available to read or download from this page.
We also share 'Jane's story'; a patient's experience of genomic testing and pharmacogenomics, and shows how knowing about genomic variation can improve care.
To join our NW Pharmacy genomics network or to give your feedback on any future events and topics that you would like to see covered, please get in touch via our contact us page.
Please bookmark this page as it will be updated regularly.
Genomic Medicine and Pharmacy Practice Educational Resources
Genomic Medicine and Pharmacy Practice Educational Resources
Jessica Keen, Pharmacy Lead for the Alliance has recently produced a 30 min education and training video. The video has been designed and developed for all pharmacy professionals, to provide an overview and insight to genomics and genomic medicine within pharmacy practice, across the NHS.
This lecture-based course from Health Education England's Genomic Education Progamme forms part of the HEE Genomics Education Programme’s Master’s in Genomic Medicine framework. It provides a comprehensive overview of the anlytical strategies and techniques used in pharmacogenomics and outlines how a patient's genetic make up can determine their response to medication.
NHS England's Genomic Education Programme offers a comprehensive range of education and training materials for professionals across multiple areas of practice.
The Centre for Pharmacy Postgraduate Education (CPPE) is part of the Division of Pharmacy and Optometry, within the Faculty of Biology, Medicine and Health at the University of Manchester.
The CPPE has a Genomics landing page with an introductory programme and signposting to other useful resources here: https://www.cppe.ac.uk/gateway/genomics
Other online resources include:
- Introduction to genomics in pharmacy CPPE Introduction to genomics in pharmacy
- NEW e-learning package from CPPE Genomics in pharmacy: an introduction to person-centred consultations Genomics in pharmacy: an introduction to person-centred consultations : CPPE
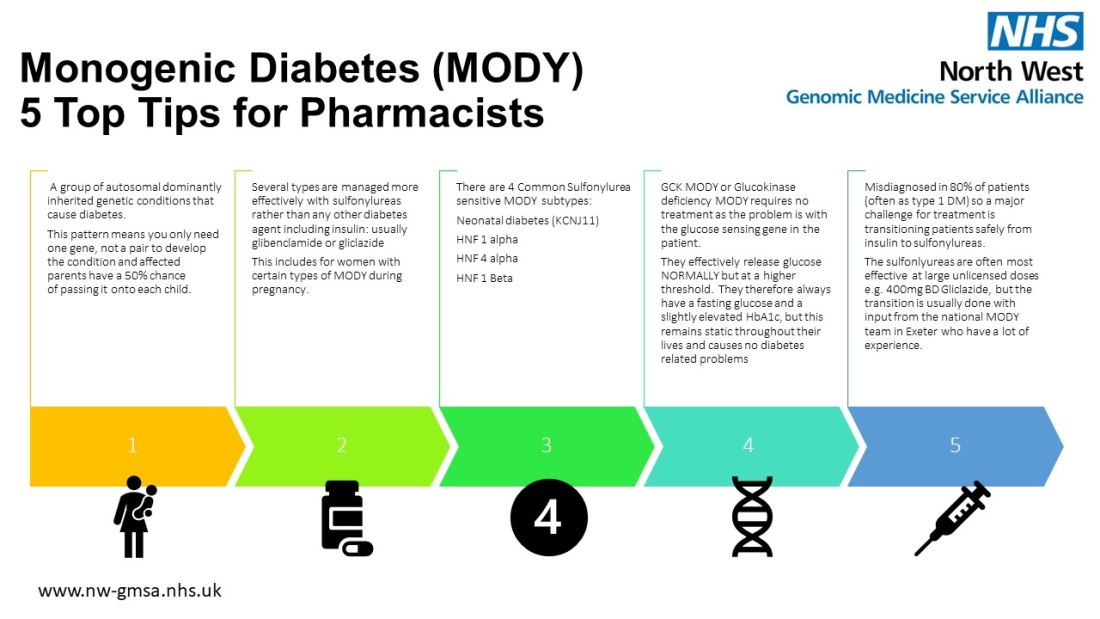
The importance of identifying monogenic diabetes can have a significant impact on the patient's treatment so that they receive the best treatment for them. For example, they may be able to take tablets instead of insulin, which can be life-changing.
Below is an infographic produced by our Monogenic Diabetes Lead, Dr. Sabnam Samad (available to download as a PDF, within our infographics page).
GeNotes is a 'just in time' resource to help healthcare professionals make the right genomic decisions at each stage of a clinical pathway.
GeNotes: Genomic notes for clinicians | GEP | Health Education England | NHS (hee.nhs.uk)
Pharmacogenomics Resources
The NHS North West Genomic Medicine Service Alliance are the lead for the national Genomic Network of Excellence: Pharmacogenomics and medicines optimisation, which will support and inform the rollout of pharmacogenomics and medicines optimisation in the NHS, including furthering the rollout in primary care.
This NHS Genomic Network of Excellence aligns with the Manchester NIHR BRC, NIHR Academic Health Science Network (AHSNs), industry partners and a Horizon 2020 Grant.
For more information on this network, please visit this page and click on the appropriate tab.
Healthcare professionals can use a patient’s genomic information to improve the safety and/ or effectiveness of the medicines they are prescribed.
This story from Jane is an example of how knowing her genomic variation could have improved the safety of her treatment and avoided a severe adverse (harmful) drug reaction.
The UK Network was set up (and is chaired) by Professor Sir Munir Pirmohamed in 2010 to develop research collaborations, between academics, clinicians, industry, and regulatory sectors.
The aim of these partnerships is to promote the use of stratified/personalised medicine in the clinic, to offer patients the most appropriate and safe drug, at the optimal dose, at the start of their treatment. Their website is a valuable resource providing information on how the field is progressing.
This tutorial which has been specially commissioned by NHS England is to support all pharmacists, pharmacy technicians and trainees working in primary and/or secondary care who currently have limited experience working with pharmacogenomic information.
The team regularly contributes and produce articles and reports which are published, primarily online. Recent examples relating to pharmacogenomics and pharmacy practice will be available online, via links shared below:
Pharmacogenetics: What pharmacists need to know article within Hospital Pharmacy Europe
https://hospitalpharmacyeurope.com/reviews-research/pharmacogenetics-what-pharmacists-need-to-know/
The Pharmaceutical Journal (pharmaceutical-journal.com)
PRSB – Guidance for Using pharmacogenomic information in clinical practice
Pharmacogenomics-Report_V1.0.pdf (theprsb.org)
British Pharmacological Society and Royal College of Physicians
Personalised Prescribing | British Pharmacological Society (bps.ac.uk)
NW Pharmacy Genomics Workforce Plan 2025-27
Regional plan to implement the National Genomic Pharmacy Workforce Strategic Framework
The National Genomics Education Programme, working with the NHS England Genomics Unit, set up the NHS England Pharmacy Workforce Group for Genomics in 2021. This group reports to the NHS England Joint Genomics Workforce Steering Group, which is led by the Genomics Education Programme and the Genomics Unit. In January 2024, the Pharmacy Genomics Workforce, Education and Training Strategic Framework was published. This framework aims to guide workforce and infrastructure planning so that genomics becomes part of everyday pharmacy practice.
The framework recommends applying the National Genomics Education Programme’s four workstreams to pharmacy over the next three years. The goal is to support all pharmacy professionals to play a key role in bringing genomic medicine into routine NHS care for patients. These four areas are:
- Raising awareness of genomics in pharmacy practice
- Building and connecting networks
- Identifying workforce needs in pharmacy genomics
- Educating and developing the pharmacy workforce
Formation of the North West (NW) Genomic Pharmacy Workforce Group: To help put this framework into practice in the North West, a short-term working group has been set up to develop and deliver a regional pharmacy genomics workforce plan. This was approved by the NW Medicines Optimisation Group and the working group first met in May 2024.
To access the NW Pharmacy Genomics Workforce Plan 2025-27 Regional plan to implement the National Genomic Pharmacy Workforce Strategic Framework document please click here.
The Royal Pharmaceutical society have issued a position statement on the role of pharmacists in the pharmacogenomics revolution. Read more here.
Our colleagues in NHS North Thames Genomic Medicine Service have produced a range of information and materials in relation to pharmacogenetics.
Pharmacogenetics study day
Podcasts
https://genomicsnow.podbean.com/
Series 4 relates to pharmacogenetics.
Featuring Vivienne Parry, Professor Bill Newman, Anita Hanson and Professor Matt Brown
www.genomicsengland.co.uk/podcasts/can-genomic-testing-prevent-adverse-drug-reactions
'Genomics in Practice' newsletters
All newsletters are available to read online or download here. We have also split out recent feature articles further down on this page.
|
In each newsletter, we use this section to highlight the role of a North West pharmacy professional working in the field of genomics or who uses genomics in their clinical practice. In this issue, Sam Alshibani - a Primary Care Network Pharmacist in Liverpool - describes how he is involved with the PROGRESS study and how genomics is used in his practice to benefit patients. The PROGRESS study overseen by the North West Genomic Medicine Service Alliance looks to bridge the gap between genetic science and frontline prescribing, an interesting concept! In my day-to-day practice, our team of Pharmacists are routinely reviewing pharmacogenetic test results, where patients are prescribed or are about to be prescribed certain medications; antidepressants, proton pump inhibitors, and statins are among the key medications under scrutiny. By personalising medication regimens to align with a patient's genetic profile, we can potentially avoid the trial-and-error approach that has been all too common in medicine and take steps to minimise potential adverse effects. In one of many instances, pharmacogenetic results allowed me to avoid under-dosing omeprazole to a patient following their hospital admission for gastritis, due to the patient’s fast metaboliser profile, they required 100% more (double) the starting dose, which goes towards helping better control their reflux symptoms, contributing to a better quality of life. Patients are not only informed about the implications of their pharmacogenetic results but are also actively involved in the decision-making process regarding their medication. This collaborative approach ensures that patients have a say in their treatment plans, fostering a sense of ownership over their health. This pharmacogenetic study could pave the way to revolutionising the way medications are prescribed. By reviewing pharmacogenetic results, evaluating prescribing decisions, and empowering patients, progress, (no pun intended) in pharmaceutical care holds immense potential for the future of healthcare, offering a glimpse into a world where treatments are truly personalised, through optimising patient outcomes, and minimising the risks associated with drug therapy.
|

In each newsletter, we use this section to highlight the role of a North West pharmacy professional working in the field of genomics or who uses genomics in their clinical practice. In this issue, Ben McIntyre - our Senior Pharmacist for genomics transformation projects – shares his path into the field of genomic medicine and describes some of the work he’s currently doing at the NWGMSA.
My interest in genomics and precision medicine began in a previous role as a specialist pharmacist in HPB (hepato-pancreato-biliary) surgery at Manchester Foundation Trust. As many will know, pancreatic cancer remains very difficult to treat and carries with it a very poor prognosis, and I became aware then of the potential for genomic tumour profiling to stratify patients and offer more personalised and effective treatments. At the same time, I recognised that medicines, in general, are not effective for many patients and that a pharmacogenomic-driven approach could significantly increase therapeutic response rates whilst reducing the likelihood of drug toxicity or adverse effects. With the advent of the new NHS Genomic Medicines Service and realising the future potential this could have for patient care, I undertook an HEE-funded MSc in Genomic Medicine part-time alongside my full-time role which I completed in 2021. This gave me an excellent foundation in all areas of genomics, including bioinformatics & technologies, cancer genomics, common & rare diseases, pharmacogenomics, infectious disease genomics, ethics, and health economics.
I took up my current post with the NWGMSA in February this year, in a part-time capacity currently split with my other role as IV and Antimicrobial Pharmacist with the Manchester Citywide IV service. Since starting, I’ve become involved in and support with several key projects and GMSA objectives, both regionally within the North West and nationally. From a pharmacy perspective, our main priority this year is the PROGRESS programme and starting the PROGRESS study as mentioned in this newsletter. This is a hugely significant piece of work, where we have a multi-disciplinary team collaborating together across clinical, informatics, laboratory, and health economic workstreams and also supporting the GPs and practice pharmacists involved in the study. Some of my other current work includes reviewing MT-RNR1 and aminoglycoside ototoxicity testing pathways across the region to support wider implementation and pharmacy workforce transformation via our genomics in pharmacy lunchtime sessions and other educational events.
This has been a truly varied and exciting role, not only where I’ve been able to use my existing skills and knowledge but it has also put me at the cutting edge of healthcare and allowed me to gain unique experience and support with wider implementation of pharmacogenomics in the NHS to deliver better care and outcomes.
In each newsletter, we use this section to highlight the role of a North West pharmacy professional who uses genomics in their practice, for the benefit of their patients. In this issue, two specialist oncology pharmacists, Ollie Williams and Joe Williams from the Christie NHS Foundation Trust explain how they use genomics to guide the treatment of patients with colorectal cancer.


Colorectal cancer is the fourth most common cancer in the UK. All patients diagnosed with colorectal cancer should undergo genomic testing of their blood and tumour tissue samples to inform the most appropriate treatment options. Identification of genomic variants can help to determine whether a patient is more or less likely to respond to certain drug treatments or whether they are at increased risk of adverse drug reactions. Most colorectal cancers are adenocarcinomas though rarely they may be squamous cell carcinomas or neuroendocrine tumours (NET) [1]. The below table provides a summary of the commonly screened genomic tests and relevant medicines available depending on the outcome of these tests.
|
Genomic test |
Status |
% Of Occurrence |
Relevant drugs |
Eligibility |
|
MMRd/MSI-H |
MSI-H MSS |
15% of Colorectal cancers have MMRd and/or MSI
|
Immunotherapy (Pembrolizumab/Nivolumab)
|
MSI-H/MMRd |
|
RAS (KRAS/NRAS) |
No mutation detected – wild type
Mutation detected
|
40% of Colorectal cancers have a KRAS mutation |
eGFR Inhibitors (Cetuximab/Panitumumab) |
Wild type |
|
BRAF V600E |
V600E mutation detected
No mutation
|
10% to 15% of Colorectal cancers have a BRAF mutation |
Encorafenib |
Mutation detected |
|
DPYD |
DPYD normal metabolizer
DPYD intermediate metabolizer
DPYD poor metabolizer |
Approximately 3% to 8% of population have partial DPD deficiency (0.01-0.5% complete) |
Fluoropyrimidines (5-FU/Capecitabine) |
Standard dosing
25% to 50% dose reduction
Avoid
|
|
MLH1 |
Indicates presence of Lynch syndrome |
10-40% |
Immunotherapy (Nivolumab/Pembrolizumab) |
MSI-H/MMRd |
|
NTRK fusion |
+ve/-ve |
<1% |
Larotrectinib |
NTRK fusion+ |
Many patients with colorectal cancers will, at some point in their treatment, receive a fluoropyrimidine based chemotherapy such as capecitabine or 5-fluorouracil. These agents are a class of chemotherapeutics which are widely used in the treatment of solid tumours, such as breast, colorectal and gastric cancers. Between 5% and 10% of patients receiving this treatment may experience neutropenia, coronary artery vasospasm, severe diarrhoea, mucositis and hand-foot syndrome [1]. 5-fluorouracil is broken down by dihydropyrimidine dehydrogenase and genetic variants in the gene encoding this enzyme (DPYD), are linked to an increase in likelihood of a patient experiencing potentially life-threatening toxicities. Patients found to have a heterozygous genotype for the DPYD gene should start therapy at a reduced dose until tolerance is assessed or use an alternative agent.
Lynch syndrome is a rare, hereditary condition which can predispose an individual to developing certain cancers including colorectal cancer. Genomic testing has enabled those patients with Lynch syndrome, and as such a higher risk of developing colorectal cancer, to be identified and treated with appropriate medications. Lynch syndrome is caused by variation in mismatch repair genes, most commonly MLH-1 and MSH-2. Tumour tissue testing is employed looking for micro satellite instability (MSI), namely MSI high which is suggestive of Lynch syndrome. If a pathological sample is found to be microsatellite stable (MSS) there is a reduced likelihood of Lynch Syndrome [2]. Patient’s identified as MSI-H become eligible for newer targeted immunotherapies such as nivolumab and pembrolizumab.
As a pharmacist screening prescriptions for colorectal cancer, it is imperative we check for the molecular profile and genetic status of each patient to ensure the most effective and appropriate therapy is being offered. As highlighted in the KEYNOTE trial, immunotherapy such as pembrolizumab confers significant increased progression free survival for patients, and with fewer adverse effects Vs conventional chemotherapy and should be offered to all patients where appropriate.
RAS and BRAF testing are completed for all patients to determine their eligibility for targeted therapies. Epidermal growth factor inhibitor (EGFRi) therapies cetuximab and panitumumab are available to those patients who show no mutation in the RAS gene (are RAS wildtype) [3]. Patients with a BRAF V600E mutation are predicted to be poor responders to EGFRi therapies [4] and so patients are generally only considered if they are also BRAF wild type. Those patients exhibiting a BRAFV600E mutation are then eligible in the second line for Encorafenib with cetuximab. In practice a delay in returning RAS/BRAF testing can impact on first line choice of chemotherapy, and we often see clinicians starting the patient on up front doublet chemotherapy in the form of irinotecan with 5-FU or oxaliplatin in combination with 5-FU, awaiting RAS/BRAF results. Once returned if the patient is found to be wild type, the EGFRi can then be added in.
Currently in practice we are seeing delays in the processing and availability of genomic testing results. Where DPYD results can be returned in less than 7 days, RAS and BRAF testing can take up to 8 weeks to complete. As per the NICE Blueteq criteria for the immunotherapy agents, the patient must be MSI-H/dMMR but we also must know the RAS status at the time of prescribing. Given the delays in returning these results it can cause a situation where a patient may not be able to have immunotherapy first line if there is a need to treat the disease without delay. Clinically there is no significant difference in outcomes for patients who are RAS wildtype or RAS mutant whilst treated with immunotherapy and because of this, discussions have taken place and a plan is pending to add a section to the Blueteq form for these drugs to state, ‘RAS status unknown’. This move will allow patients to commence immunotherapy whilst RAS results are still pending[5].
References:
- Colorectal cancer, GeNotes – Genomic Notes for Clinicians: https://www.genomicseducation.hee.nhs.uk/genotes/knowledge-hub/colorectal-cancer/
- British Society of Gastroenterology, 2022. Available: A brief guide to the management of Lynch Syndrome - The British Society of Gastroenterology (bsg.org.uk)
- MHRA drug safety updates (2014), Cetuximab. Available:https://www.gov.uk/drug-safety-update/cetuximab-new-safety-information-available
- Targeted Therapy for Colorectal Cancers With Non-V600 BRAF Mutations: Perspectives for Precision Oncology | JCO Precision Oncology (ascopubs.org)
- NICE TA709 (2021)
https://www.ncbi.nlm.nih.gov/books/NBK1211/
In each newsletter we use this section to highlight the role of a North West pharmacist who uses genomics in their practice, for the benefit of their patients. In this issue, Alice Binns the Senior Antimicrobials Pharmacist based at The Northern Care Alliance (NCA) tells us how genomic testing is used within their service.

At The NCA a proportion of our positive influenza samples are sent for viral DNA sequencing. This information is shared with UKHSA who collate and publish weekly national surveillance reports detailing numbers and proportion of positive results for each of the current Influenza subtypes; A(H3N2), A(H1N1)pdm09 and B. Surveillance monitoring can allow us to adjust influenza treatment if the dominant circulating strain shows resistance to oseltamivir (Tamiflu). If this was found to be the case, we would use zanamivir first line instead. In the past A(H1N1)pdm09 has shown resistance to oseltamivir however so far this season in the UK we have not found any reduced susceptibility which is reassuring.
In a recent C Diff. RCA Scrutiny Panel at The Royal Oldham, we had a case which involved someone who had been nursed in a side room which had previously been used by another patient who had also tested positive for CDT. In a scenario like this it is important to question whether cross transmission between two patients could have occurred. In this case we were able to send both patients’ isolates for ribotyping using the enhanced fingerprinting service to determine the C Diff strain causing the infection. It was found that both isolates were in fact the same strain which led the panel to deduce that the second case had almost certainly been a result of cross-transmission and should have been avoidable.
A recent topic of discussion at our NCA weekly microbiology Journal Club was the R65 genomic test for the m.1555A>G variant which is associated with an increased risk of aminoglycoside-associated ototoxicity. This started some interesting conversations around the evidence for the increased risk, available national guidance, accessibility and feasibility of the test and potential cohorts of patients who might benefit from being offered this. We also began to discuss what a positive test result might mean for patients in terms of treatment options. These discussions are now ongoing!
In each newsletter, we will use the Genomics in Practice section to highlight the role of a North West pharmacist who uses genomics in their practice to the benefit of their patients.
In the September 2022 edition, Michael Dooney, the Adult Cystic Fibrosis (CF) Pharmacist based in Blackpool, tells us how genomic testing is used within their service.

Nowadays people are typically, although not exclusively, diagnosed with CF via the NHS new-born screening programme. Before the inception of the national screening programme failure to thrive, loose oily stools and recurrent chest infections in children were presentations that were potentially indicative of CF and resulted in referral for testing. Those who screen as potentially having CF through the immunoreactive trypsin test have their blood spot sent for genetic testing to look for variants in the CFTR gene and are referred for a sweat test. The CFTR protein is responsible for chloride and bicarbonate transport across epithelial surfaces in the airway, gastrointestinal and reproductive tracts, pancreas, and sweat glands. Genetic changes in the CFTR protein can lead to an absence or reduction in the quantity or function of CFTR, or both. This dysfunctional anion transport results in viscous mucus secretions, that are characteristic of CF, and multiorgan dysfunction, including pancreatic insufficiency and airway infection and obstruction. Chronic airway infection leads to progressive lung damage and eventually respiratory failure.
Traditionally medical management has been based on the treatment of the downstream effects of CF, i.e., symptom management. This resulted in a high burden of care which included: high use of oral and nebulised antibiotics, nebulised mucolytics, bronchodilators, daily airway clearance therapy, pancreatic enzymes, fat soluble vitamin supplements amongst many others and multiple admissions for rescue treatment with IV antibiotics. Whilst great strides have been made in the medical management of CF over the years it was reported in 2018 that approximately half of those born with CF born that year were expected to live into at least their mid-40s.
In 2013 a new, first of its kind, treatment for CF was made available through the NHS called ivacaftor (Kalydeco®). This treatment, a CFTR modulator, was revolutionary in that it was the first treatment that targeted the genetic problem of CF at a protein level rather than the sequelae of CF. It helped to restore CFTR protein functionality to enough of a degree to produce some dramatic results. In trials participants saw significant improvements in lung function, the need for both oral and IV antibiotics reduced due to reductions in pulmonary exacerbations, as well as improvements in quality-of-life scores. It is a truly life changing drug. Sadly, it was only effective for approximately 4.5% of the CF population of the UK due to the prevalence of the mutations it was effective for in the country. Therefore, it was only prescribed for individuals with one copy of the following mutations: G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N or S549R
New CFTR modulators, tezacaftor/ivacaftor (Symkevi®) and lumacaftor/ivacaftor (Orkambi®), became available in 2019 that were targeted at a wider range of the CF population. These were approved for use in those with CF who were homozygous for the F508del mutation, which is the most common CF genotype. This meant that it could be prescribed for approximately 50% of the CF population in the UK. It also provided similar health benefits to ivacaftor in that it improved lung function, reduced need for antibiotics due to pulmonary exacerbations of CF and improved quality of life scores but the improvements were not as drastic as they were for ivacaftor in those with the gating mutations listed earlier. However, it represented a significant step forward in the care of those with 2 copies of the F508del mutation.
Then in 2020 elexacaftor/tezacaftor/ivacaftor (Kaftrio®) was approved for use in the NHS for those with one copy of the F508del mutation, which represented approximately 90% of the CF population. This drug has truly revolutionised CF care as it has brought dramatic ivacaftor for gating mutations-like improvements in lung function, exacerbation reduction and improvements in quality of life to the masses. There are numerous life changing stories due to the effect Kaftrio® on the lives of those with CF who can benefit from his treatment and the outlook for the future has never looked so bright for those who fit these genetic criteria with life expectancy greatly improved, if yet unquantifiable due to the newness of these treatments resulting in this data not currently being available.
Genetic testing provided by the NW Genomic Laboratory Hub, as per criteria set out in the National Genomic Test Directory, gives information about the exact type of variant in the individual patient. This information was critical in helping us to identify those patients who are eligible for these life changing treatments. As the adult CF lead pharmacist, I have been responsible for checking all the genetic reports for our population to ensure they are on the most effective treatment available and rechecking eligibility for treatments as new combinations of CFTR modulators have been approved. The use of drugs informed by genomics has drastically improved the care of those with CF.
Without a doubt it has been one of the biggest steps forwards in the medical management in the history of CF, if not the biggest.
We are now looking to further incorporate genomics into our practice on the back of the success of the rollout of CFTR modulators by testing for genetic sensitivity to aminoglycoside-induced hearing loss by screening for the m.1555A>G mitochondrial mutation in our population.
Acknowledgements: Health Education England's Genomic Education Programme, Health Education England, UK Pharmacogenetics & Stratified Medicine Network, CPPE, NHS North Thames Genomic Medicine Service Alliance, and The Royal Pharmaceutical Society.
